Abstract
Cognitive-behavioral therapy (CBT) is effective for pediatric obsessive-compulsive disorder (OCD), but non-response is common. Brain glutamate (Glu) signaling may contribute to OCD pathophysiology and moderate CBT outcomes. We assessed whether Glu measured with magnetic resonance spectroscopy (MRS) was associated with OCD and/or CBT response. Youths aged 7–17 years with DSM-IV OCD and typically developing controls underwent 3 T proton echo-planar spectroscopic imaging (PEPSI) MRS scans of pregenual anterior cingulate cortex (pACC) and ventral posterior cingulate cortex (vPCC)—regions possibly affected by OCD—at baseline. Controls returned for re-scan after 8 weeks. OCD youth—in a randomized rater-blinded trial—were re-scanned after 12–14 weeks of CBT or after 8 weeks of minimal-contact waitlist; waitlist participants underwent a third scan after crossover to 12–14 weeks of CBT. Forty-nine children with OCD (mean age 12.2±2.9 years) and 29 controls (13.2±2.2 years) provided at least one MRS scan. At baseline, Glu did not differ significantly between OCD and controls in pACC or vPCC. Within controls, Glu was stable from scan-to-scan. Within OCD subjects, a treatment-by-scan interaction (p=0.034) was observed, driven by pACC Glu dropping 19.5% from scan-to-scan for patients randomized to CBT, with minor increases (3.8%) for waitlist participants. The combined OCD participants (CBT-only plus waitlist-CBT) also showed a 16.2% (p=0.004) post-CBT decrease in pACC Glu. In the combined OCD group, within vPCC, lower pre-CBT Glu predicted greater post-CBT improvement in symptoms (CY-BOCS; r=0.81, p=0.00025). Glu may be involved in the pathophysiology of OCD and may moderate response to CBT.
Similar content being viewed by others
Introduction
Both medication and cognitive-behavioral therapy (CBT) are efficacious for OCD, but elicit inadequate response in 40–60% of patients (McGuire et al, 2015). Moreover, effective response predictors are lacking (Torp et al, 2015), reflecting incomplete understanding of OCD pathophysiology. The need to identify subgroups for treatment matching and response prediction has intensified as novel treatments are proposed. Most relevant research has focused on adults, but childhood-onset OCD is distinct (Geller, 2006), warranting separate study.
Incomplete response to serotonin-reuptake inhibitors has led researchers to consider other signaling pathways. Converging evidence links OCD with central glutamate (Glu) (Rosenberg and Keshavan, 1998). Several Glu-related genes show tentative associations with OCD (Arnold et al, 2009; Wu et al, 2012) and two studies reported above-normal CSF Glu in OCD (Chakrabarty et al, 2005; Bhattacharyya et al, 2009). Improvement in refractory OCD following Glu-modulating drugs has intensified interest in Glu and treatment response (MacMaster et al, 2008; Pittenger et al, 2006, 2011). Response to these agents varies across patients and there is a need to identify sources of this variation to inform possible future personalized interventions.
Interest in in vivo OCD pathophysiology has spurred studies using magnetic resonance spectroscopy (MRS) to assay brain Glu, its precursor glutamine (Gln), or their sum (Glx) (Brennan et al, 2013). Brain regions in putatively symptom-driving cortico-striatal-thalamo-cortical circuits have been studied, including caudate, orbitofrontal cortex, and pregenual anterior cingulate cortex (pACC). Results have varied, possibly influenced by demographics (pediatric vs adult), region assayed, medication, and MRS procedures. Moreover, most investigations are underpowered and pediatric studies are sparse.
The few pediatric studies have reported above-normal caudate (Rosenburg et al, 2000) and pACC (O'Neill et al, 2012) Glx, although another report found below-normal pACC Glx (Rosenburg et al, 2004). An additional study detected no differences in Glx vs controls (Ortiz et al, 2015), but a high rate of pharmacotherapy (80%) complicated interpretation. In adults, two reports indicated elevated Glu in OCD (O'Neill et al, 2016; Zurowski et al, 2007), whereas others do not (Simpson et al, 2012; Brennan et al, 2015; O'Neill et al, 2013; Starck et al, 2008). Some studies, in adults or children, revealed correlations between OCD symptom severity and Glx, in the absence of mean differences between OCD and controls (O'Neill et al, 2012; Stark et al, 2008; Yücel et al, 2008). The present study examined largely drug-free pediatric OCD patients.
Supporting a role for Glu in OCD treatment response are reports identifying Glx reductions after paroxetine (Rosenburg et al, 2000) or CBT (Zurowski et al, 2007; O'Neill et al, 2013). Although Glx changes post-CBT were absent in another study, the authors noted the sample’s lower pretreatment severity and Glx concentrations compared with their prior paroxetine trial (Benazon et al, 2003). Overall, data supporting glutamatergic causation of OCD are mixed, but other data suggest that Glu regulation in OCD relates to symptom severity and treatment response and specifically that reductions in Glu are linked to symptomatic improvement with CBT. The present investigation examined this possible CBT–Glu association.
Methodological challenges such as low field strength and partial voluming have prevented reliable separation of Glu from the larger Glx signal or the assignment of Glu effects to specific brain regions, preventing definitive conclusions on the role of Glu per se in OCD and treatment response. To address these issues, the present study employed multivoxel proton echo-planar spectroscopic imaging (PEPSI) MRS. PEPSI at 3 T effectively quantifies Glu and enables brain sampling with 0.5-cc voxels at 15-ms echo-time (TE) (Posse et al, 2007; Zhang, 2013). In addition, as models of OCD increasingly emphasize affective dysregulation in regions beyond cortico-striatal circuits (Milad and Rauch, 2012), we interrogated Glu in another region, ventral posterior cingulate cortex (vPCC), in a subsample of our data set. pACC and vPCC may co-mediate emotion regulation (Vogt, 2009), and recent morphometric and fMRI findings implicate vPCC in OCD (Cheng et al, 2013; Tang et al, 2013; Ciesielski et al, 2012; Brennan et al, 2016).
Our investigation addressed three questions: Do pretreatment Glu levels differ between mostly drug-free pediatric OCD and controls? Do these levels change after CBT? And, does baseline Glu predict post-CBT response? CBT has established efficacy as a first-line pediatric OCD treatment (Piacentini et al, 2011), and our preliminary results in pediatric (O'Neill et al, 2012) and adult (O'Neill et al, 2013) OCD identified possible effects of CBT on MRS metabolites, including Glx decrease in anterior cingulate. We hypothesized a pre-CBT excess of Glu in OCD patients vs controls that lessens after CBT and that individual Glu levels help explain variability in CBT response.
Materials and methods
This study was a randomized, waitlist-controlled, crossover trial of CBT for OCD, combined with multiple MRS acquisitions. After screening for eligibility, OCD and healthy control children were enrolled by clinical research staff and scanned with MRS by operators blind to diagnosis. OCD participants were then randomized 1:1 to an ‘active CBT’ or an initial ‘waitlist’ arm. Randomization was performed by the UCLA Semel Institute Statistics Core using randomized permuted blocking with block size four and covariate adaptive randomization for medication status, gender, and age. Randomization assignment was kept in a sealed envelope opened shortly before commencing treatment. Participants in the active CBT arm received 12–14 sessions of weekly standardized CBT (Piacentini and Roblek, 2007), upon completion of which they underwent a second MRS scan; participants randomized initially to the waitlist condition received no intervention for 8 weeks, after which they underwent a second scan. Subsequently, they crossed over to 12–14 weeks of CBT and then completed a third scan. Controls were scanned twice; once after screening, and again after 8 weeks of no intervention to afford assessment of MRS Glu scan–rescan reliability.
Participant Selection
Prior to research procedures, written informed consent was obtained from parents and written assent from children (⩾8 years). The setting was a University-based medical center (UCLA) and the study was approved by the UCLA Human Subjects Protection Committee. Target sample size was based on attaining 80% power at α=0.05 for post-CBT reduction in pACC Glu, based on our pilot data.
Participants were recruited by referral from UCLA psychiatric and pediatric clinics, other local clinics, and private psychiatrists and psychotherapists, as well as by flyers, radio and Internet ads, and word-of-mouth. Inclusion criteria for OCD participants included: (1) males or females aged 7–17 years; (2) a primary DSM-IV diagnosis of OCD per the Anxiety Disorders Interview Schedule-Research Lifetime Version (ADIS-RLV); (3) Children’s Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) score ⩾16 (clinically significant impairment); (4) demonstrated ability to cooperate with study procedures and participate in CBT in the judgment of the study clinician; (5) no psychotropic medication or stable concurrent psychotropic medication for a minimum of 12 weeks prior to screening and no anticipated need to change dose or treatment during the study; and (6) IQ ⩾80 on the Wechsler Intelligence Scale For Children (WISC). Exclusion criteria included: (1) lifetime DSM-IV diagnosis of pervasive developmental disorder, mania, psychotic disorder, conduct disorder, or substance dependence; and (2) failure of prior adequate (>10 sessions of therapist-directed exposure-based treatment) CBT. Inclusion criteria for healthy controls were: (1) males and females aged 7–17 years; (2) IQ ⩾80 on the WISC; and (3) no current or lifetime Axis I psychiatric disorder per ADIS-RLV. Receipt of prior adequate CBT (>10 sessions of therapist-directed exposure-based treatment) was a study exclusion. Thus the sample was was not treatment refractory with regard to CBT. Prior medication history and, hence, treatment refractoriness for medication, were unknown, except for four still symptomatic cases enrolled on stable (⩾12 weeks) medication doses: one each with lorazepam, sertraline, fluoxetine plus guanfacine, and lisdexamfetamine; thus no participants being treated with Glu modulators were included; study results were essentially unchanged when medicated participants were excluded.
CBT and Waitlist Procedures
Participants randomized to active CBT received 12–14 sessions of weekly, manualized CBT (Piacentini and Roblek, 2007), which focused on exposure plus response prevention but included other elements standard to the treatment of OCD in children and adolescents, including psychoeducation, cognitive strategies, daily homework, and parent and family involvement. All study therapists completed extensive training and supervised therapy of at least two cases using the study CBT protocol prior to treating any study patients. After each session, the CBT therapist completed a standardized session summary form outlining the events of that session and assessing homework completion and quality since the last visit. Therapists also completed a treatment fidelity checklist indicating the extent to which they had followed the treatment manual for that session and providing an explanation for any protocol deviations (which were rare). Therapists received weekly group supervision from JP during which each study therapy session, along with associated patient compliance and therapist fidelity ratings, were reviewed. Within 1 week of concluding CBT, participants underwent a second MR scan, as well as posttreatment clinical assessments. Participants randomized initially to the 8-week waitlist condition received one brief (non-therapeutic) telephone call every other week to ensure continued study participation. Upon completing waitlist period, they underwent MR scanning and clinical assessments before crossing over and beginning an identical 12–14-session CBT regimen. Upon completing CBT, they underwent a third scan and clinical assessments.
Clinical Assessments
All assessments were performed by independent evaluators (IEs) blinded to treatment condition and study visit. Interviewer and therapist training and supervision procedures were based on those successfully used in prior studies from our group (including Piacentini et al, 2011). All IEs underwent rigorous training, including agreement with senior staff (SC, TP) ratings on diagnosis, CY-BOCS, and Clinical Global Impressions (CGI) scores on at least two training cases. All baseline and follow-up assessments were discussed during weekly supervision with senior staff. IE assignments were varied so as not to unblind evaluators through the difference in time between the active CBT and waitlist conditions. Primary and secondary endpoints of CBT response were: (1) CY-BOCS Total Score; and (2) Clinical Global Impressions-Improvement (CGI-I). CY-BOCS and CGI-I were obtained at screening, baseline (randomization) and end of first study phase (8 weeks for waitlist; 12–14 weeks for CBT). For participants initially randomized to waitlist and crossing over to CBT, assessments were repeated a third time at the end of CBT.
Magnetic Resonance Acquisition
Whole-brain MRI and PEPSI MRS were acquired on a 3 T Siemens Trio with 12-channel phased-array head coil. Structural MRI was acquired with an axial-oblique (genu-splenium) magnetization-prepared rapid gradient-echo (MPRAGE, repetition-time (TR)/TE=1900/2.26 ms, 1 × 1 × 1 mm3) volumetric scan. This MPRAGE and ‘coronal’ and ‘sagittal’ resliced copies thereof were used to prescribe MRS. A pediatric neuroradiologist (NS) reviewed MRIs to exclude subjects with clinical abnormalities.
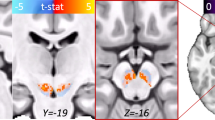
Multivoxel water-suppressed PEPSI (TR/TE=2000/15 ms, 8 excitations, matrix 32 × 32, voxels 7.6 × 7.6 × 9 mm3=0.52 cc, 8:32 min; Posse et al, 2007) was acquired from an axial-oblique slab parallel to the MPRAGE. Bilateral pACC (Figure 1) was sampled from a localized shim volume at the slab center. Rather than the single voxel of localized MRS methods, PEPSI acquires an entire grid of voxels simultaneously. A limitation is that the voxel boundaries are not as sharply defined as for single voxels, ie, metabolite signals can leak to adjacent voxels. In particular, voxels near the skull are subject to extracranial lipid contamination. For this reason, the PEPSI slabs were positioned well away from the edge of the brain. The shim volume was positioned laterally symmetrically at the dorsal–ventral midpoint of the genu corpus callosum and ran anterior from there to the frontal pole without contacting extra-brain tissue. Only caudomesial portions of the box contained pACC, more anterior was superior frontal cortex, and more lateral prefrontal white matter (anterior corona radiata). In a portion of participants (20 OCD, 12 controls), bilateral vPCC was sampled from the posterior end of an additional PEPSI slab with identical orientation (Figure 1). vPCC was located just caudal to the splenium of the corpus callosum. Identical non-water-suppressed PEPSI volumes (one excitation) were acquired immediately before the pACC and vPCC slabs for purposes of offline metabolite quantification.
The left upper and lower panels feature sagittal and the right upper and lower panels axial-oblique whole-brain T1-weighted MRI showing prescription of 9-mm thick proton echo-planar spectroscopic imaging (PEPSI) MRS slabs (fine white lines). Upper panels show sampling of bilateral pregenual anterior cingulate cortex (pACC) and the lower panels ventral posterior cingulate cortex (vPCC). The 32 × 32 grids of 7.6 × 7.6 mm2 voxels are not shown, nor the 7 saturation bands arrayed on all sides to suppress extracranial lipids during acquisition. Thick white lines mark the ‘shim boxes’, target regions where the B0 field is homogenized prior to excitation. The pACC shim box is laterally symmetric at the dorsal–ventral midpoint of the genu corpus callosum and runs thence anterior to frontal pole. Caudomesial portions of the box sample pACC. The vPCC is sampled caudal to splenium corpus callosum within a larger shim box that also interrogates basal ganglia, thalamus, and posterior white matter. The center panel shows a sample PEPSI MR spectrum from pACC with the prominent glutamate–glutamine complex (‘Glu+Gln’) labeled.
Magnetic Resonance Postprocessing
All postscan processing was performed by staff blinded to participant diagnosis and treatment condition. Each MPRAGE was segmented into gray matter, white matter, and CSF subvolumes using FSL FAST. Further, each MPRAGE was parcellated into regional volumes-of-interest (VOIs) using FreeSurfer v5.1. These included a ‘pACC’ VOI corresponding to the FreeSurfer ‘rostanteriorcingulate’ label and a ‘vPCC’ VOI, which was the ventral portion of the FreeSurfer ‘isthmuscingulate’ label (Desikan et al, 2006). Each tissue subvolume and VOI was converted into a binary mask and reconstructed in the native space of each MRS voxel using the SVFit2013 update to our MVP software suite (Seese et al, 2011). This software calculated the volume percentage of each tissue subvolume and VOI in each MRS voxel and corrected MRS metabolite levels for voxel CSF content.
MR spectra were fit with LCModel yielding levels of Glu and the other proton neurometabolites N-acetyl-aspartate+N-acetyl-aspartyl-glutamate, Glx, creatine+phosphocreatine (Cr), choline-compounds (Cho), and myo-inositol (mI), as well as lipids and macromolecules, all referenced to unsuppressed water and expressed in Institutional Units (IU). Spectra with obvious artifact (head motion, lipid contamination, inadequate water suppression, eddy currents not removed by LCModel) or poor quality (linewidth >0.1 ppm, signal-to-noise <4) were rejected in toto. Glu and Gln were separated per the LCModel fitting algorithm, as this was shown to deliver reliable values for PEPSI in multisite human brain field testing (Posse et al, 2007) and in systematic human and phantom pulse-sequence fitting comparison studies (Zhang, 2013). Values for Glu were rejected if the Cramer–Rao Lower Bound of the spectral fit exceeded 20%. A representative Glu level for left pACC, for example, was obtained by averaging together the values for all voxels with ⩾50% VOI content of left pACC; an identical method was used for right pACC and for left and right vPCC. Metabolite levels for the left and right hemispheres for both pACC and vPCC were then averaged as there were no significant hemispheric effects in either group.
Statistical Analyses
Demographic differences between both diagnostic (OCD vs controls) and treatment condition (CBT vs waitlist) groups were examined by t-test or χ2 tests to determine comparability. Response to intervention (CBT or waitlist) for OCD symptoms was tested via paired t-tests comparing pretreatment vs posttreatment CY-BOCS Total Scores or via single-sample t-tests comparing CGI-I scores to the fixed value 4 (‘no change’). Using conventional definitions, responders to treatment were defined as demonstrating ⩾35% posttreatment decrease in CY-BOCS from baseline (Goodman et al, 1993). In line with prior trials (eg, Piacentini et al, 2011), an additional measure of response was applied defined as a posttreatment CGI-I score of 2 (‘much improved’) or 1 (‘very much improved’).
MRS analyses focused on a single metabolite (Glu) in two VOIs, pACC and vPCC, the latter being exploratory. Given the small number of tests performed, the a priori nature of two of the hypotheses, and the exploratory nature of the third, α was set at 0.05 without correction for multiple comparisons. First, to test for possible diagnostic group differences, mean Glu levels in each VOI were compared between OCD and control samples at baseline using independent t-tests. To determine test–retest reliability of MRS Glu, levels within the control group were compared at the baseline and 8-week scans using paired t-tests. To test possible effects of CBT on Glu, repeated-measures analysis-of-variance (R-ANOVA) was conducted across the active CBT and waitlist OCD arms comparing the first two MRS scans. The measure was Glu and one R-ANOVA was performed for each VOI. Age and sex were found to influence neither clinical end points nor Glu levels and thus were excluded from the model. With Scan as within-subjects factor (two levels: baseline, post-CBT or post-waitlist) and Treatment as between-subjects factor (two levels: CBT, waitlist), the Treatment-by-Scan interaction was examined to determine differential effects of treatment condition on Glu concentrations from pretreatment to posttreatment. Next, aggregating patients from the active CBT and waitlist arms after CBT crossover, R-ANOVA was again applied comparing pre- and post-CBT Glu for the combined group. Finally, to examine possible prognostic effects of regional Glu on CBT outcome, the Pearson correlation was examined between baseline Glu in pACC and vPCC and %change in CY-BOCS (100 × (post−pre)/pre). Mean regional Glu was also compared between OCD responder and non-responder subgroups with independent t-tests.
Results
Participants were recruited, treated, and scanned between January 2009 and February 2014 when cohorts were complete. After baseline scan, OCD participants were randomized to active CBT (30 participants) or minimal-contact waitlist (30 participants). Eleven participants had no MRSI data from the target pACC or vPCC brain regions, therefore these subjects were excluded and further analysis was based on the 49 participants (24 CBT/25 waitlist) who did. Similarly, of 35 typically developing controls receiving baseline scan, 6 had no MRSI data from the pACC or vPCC and analysis was based on the remaining 29 who did. MRS data from 49 OCD (22 girls/27 boys, mean age 12.2±2.9 years) and 29 control (14 girls/15 boys, 13.2±2.2) participants were acquired (Table 1, CONSORT diagrams in Supplementary Information). Mean CY-BOCS at intake was 23.9±3.7, indicating moderate-to-severe impairment in most participants. Expected comorbidities (Table 1) were moderately represented in the OCD sample, including anxiety disorders, eating disorders, mood disorders, tic disorders, and attention-deficit/hyperactivity disorder.
Effects of OCD Diagnosis on Glu
Usable data for the analysis of baseline Glu across diagnostic groups were available in the pACC for 39 (18 boys/21 girls, 12.1±3.0) of the original 49 OCD and 26 of the 29 control (13 boys/13 girls, 13.3±2.3) participants and in the vPCC for 15 of the 20 OCD (7 boys/8 girls, 10.8±2.3) and 12 of the 12 control (6 boys/6 girls, 12.3±2.7) participants. Sex and age of these subsamples did not differ significantly from their respective original samples. Baseline mean Glu for OCD participants (pACC: 13.2±2.9 IU; vPCC: 13.1±4.6 IU) did not differ significantly from the corresponding values in healthy controls (pACC: 12.7±3.3 IU, p=0.576; vPCC: 11.3±2.8 IU, p=0.228).
Response to CBT
Forty-four children were followed to study completion (for subject disposition, see CONSORT, Supplementary Information). For the overall OCD sample, mean CY-BOCS dropped 42.8% to 13.0±9.3 (p=9E-12) post-CBT, against 8.6% post-waitlist (p=0.018). Mean CGI-I was 2.2 (‘2’ equals ‘much improved’, ‘3’ equals ‘minimally improved’) post-CBT (p=0.0005) and 4.2 (‘4’ equals ‘no change’, ‘5’ equals ‘minimally worse’) post-waitlist (p=0.397). Based on a ⩾35% reduction in CY-BOCS criterion, 30 (68.2%) participants were classified as responders and 14 (31.8%) as non-responders. Results were highly similar when defining responder by CGI-I. Five participants terminated the study early (one withdrew prior to waitlist, two began non-study treatment during waitlist, one started non-study treatment during active CBT, and one began non-study treatment during crossover CBT; CONSORT).
Effects of CBT on Regional Glu
In the pACC, of the original 49 OCD participants, 32 (13 girls/19 boys, 11.7±2.9) had usable Glu data for the analysis of the effects of treatment. In the vPCC, of the original 20 OCD participants, 15 (7 girls/8 boys, 10.7±2.6) had usable Glu data. Sex and age of these subsamples did not differ significantly from their respective original samples. R-ANOVA revealed a significant Treatment-by-Scan interaction for Glu in pACC (F1,31.6=4.9, p=0.034; Figure 2) but not in vPCC. pACC Glu decreased significantly (19.5%) for participants undergoing active CBT but not for participants on waitlist (3.8% increase). Combining pre–post-CBT data for the active CBT and initial waitlist group after CBT revealed a highly significant post-CBT reduction in pACC Glu (16.2%; F1,24.6=10.0, p=0.004; Figure 2). In contrast, no treatment effects on Glu were observed in vPCC. Glu levels in controls remained stable over a parallel period between baseline (pACC: 12.7±3.3 IU, p=0.149; vPCC: 11.3±2.8 IU) and second (13.9±3.8 IU in pACC; 11.6±3.1 IU in vPCC) scan (p=0.500).
Within the pediatric obsessive-compulsive disorder sample, the left panel shows that participants randomized to active cognitive-behavioral therapy (CBT; filled circles) experience a notable posttreatment (Scan 2 vs Scan 1) drop in glutamate (Glu) levels in pregenual anterior cingulate cortex (pACC), while participants randomized to waitlist (open circles) do not (interaction p=0.034). Waitlist participants subsequently crossed over to undergo the same CBT regimen. When prepost CBT data for these crossover participants are combined with those for active CBT participants (right panel), a significant post-CBT reduction in pACC Glu was again registered (16.2%; F1,24.6=10.0, p=0.004).
Effects of Baseline Regional Glu in Predicting CBT Response
Within our limited OCD subsample with vPCC MRS data, pretreatment Glu was significantly and positively correlated with post–pre-CBT %change in CY-BOCS total score (r=0.81, p=0.00025; Figure 3), indicating that lower baseline Glu was associated with greater clinical response. Mean baseline Glu was 31.4% lower for responders (11.0±3.2) than for non-responders (18.8±1.8, p=0.00015) when responder status was based on ⩾35% posttreatment CY-BOCS decrement. Results were similar when using CGI-I response criteria (Figure 3). In pACC, baseline Glu did not predict CBT response.
Left panel: In pediatric obsessive-compulsive disorder (OCD) participants undergoing cognitive-behavioral therapy (CBT), glutamate (Glu) levels in ventral posterior cingulate cortex (vPCC) at pretreatment baseline correlated positively (Pearson r=0.81, p=0.00025) with post–pre-CBT %change in Child Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) total score, reflecting severity of OCD core symptoms. Red circles denote patients defined as ‘Responders’ to CBT based on experiencing a post-CBT drop of ⩾35% in CY-BOCS and a Clinical Global Impression—Improvement (CGI-I) score of 2 (‘much improved’) or 1 (‘very much improved’); yellow circles denote patients who are ‘Non-responders’ based on these criteria; striped circles denote patients who are Responders per CY-BOCS but not CGI-I. Right panel: pre-CBT vPCC Glu was significantly lower for patients who later responded (per CGI-I) to CBT (group-mean 10.2±3.0 IU) than for those who failed to respond (17.4±2.7 IU) to 12–14 weeks of weekly CBT (p<0.0005). Similar results were obtained when using a post-CBT drop of ⩾35% in CY-BOCS score as criterion for response. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Discussion
This randomized controlled clinical trial represents one of the largest studies to date of the effects of CBT on brain Glu in a mostly unmedicated pediatric OCD population. Methodological innovations included smaller voxels and shorter TE and interrogation of the vPCC, a region seldom examined in OCD. There were two major findings: (1) pACC Glu decreased significantly in OCD after CBT and (2) higher vPCC Glu at pre-CBT baseline predicted poor response to CBT. Taken together, these findings add support for glutamatergic theories of OCD therapeutics (Rosenberg and Keshavan, 1998; Pittenger et al, 2006; Carlsson, 2000; O'Neill et al, 2013). However, in our large sample of mostly treatment-naive youth with OCD, we did not confirm a hypothesis of elevated baseline Glu in OCD vs controls in the two cingulate subregions queried. Therefore, these results imply more complex relations between Glu and therapeutic response than a straightforward ‘abnormal elevation decreasing after treatment’.
The finding that pACC Glu decreased in OCD participants after CBT is strengthened by the lack of change in Glu in waitlist participants and in healthy control children, who both underwent rescanning after an 8-week interval. Furthermore, Glu reduction in waitlisted OCD participants after crossover to CBT was similar to that in participants randomized initially to CBT. These findings support the conclusion that the observed reduction in Glu with CBT is linked to processes underlying symptom reduction, as opposed to being an artifact of rescanning or habituation to procedures. It is difficult to compare our findings directly with those of prior MRS studies in pediatric and adult OCD, as those studies used larger voxels and longer echo-times and most reported results as Glx rather than Glu. Our failure to find abnormal Glu in pACC in pediatric OCD is, however, consistent with most of these studies (Ortiz et al, 2015; Simpson et al, 2012; Brennan et al, 2015; O'Neill et al, 2013; Starck et al, 2008; Lázaro et al, 2012). Assay of Glx rather than Glu may also explain the absence of effects of CBT in the caudate in a prior pediatric study (Benazon et al, 2003). However, our results are consistent with a non-significant trend for reduction in caudate Glx in nine pediatric OCD participants (Whiteside et al, 2012), and in adult OCD, where we observed post-CBT reduction in Glx in anterior middle cingulate (O'Neill et al, 2013). Therefore, present results offer perhaps the best evidence to date for CBT-related reduction of Glu in anterior cingulate in OCD. As baseline Glu was not elevated in OCD patients, it is possible that CBT is not normalizing dysregulated Glu but rather is producing a compensated state. Resting-state metabolic activity of the brain is linearly coupled to its neuronal activity (Hyder et al, 2013), reflecting the actions of glutamatergic neurons (Hyder et al, 2006; Rothman et al, 2011). Changes in Glu could be a secondary consequence of primary alterations in neural activity. This underlines the importance of conducting MRS and/or other neuroimaging in conjunction with clinical trials of putative Glu-modulatory therapies for OCD, in the attempt to identify core mechanisms of response.
Our data affirm recent models (Milad and Rauch, 2012) emphasizing a role for ACC in OCD. Cingulotomy yields ⩾35% reduction in symptoms in ~50% of treatment-refractory OCD patients and deep brain stimulation of the ventral striatum may alleviate OCD via its connections to ACC. Neuroimaging also links the ACC to CBT response. BOLD activation in ACC during symptom provocation diminishes after CBT in adult OCD (Nakao et al, 2005). Pretreatment hyperactive ACC BOLD responses to personalized obsession provocation began to diminish within the first weeks of CBT (Morgiève et al, 2014). Lower pre-CBT cortical thickness of pACC correlated with post-CBT symptom reduction and predicted treatment responders (Fullana et al, 2014). This collective evidence suggests that ACC physiology influences receptivity to CBT and reciprocally can be altered by CBT. ACC hyperactivity (elevated PET cerebral blood flow and glucose metabolism; Saxena et al, 2009a, 2009b) has been interpreted as evidence of ACC dysfunction. Furthermore, inability of the ACC to extinguish activation may allow excessive activity to persist. Changes observed in Glu associated with CBT and symptom improvement may reflect restoration of normal ACC function. Thus plausible mechanisms have been offered to connect ACC with CBT response and should motivate additional research. Further MRS investigation of Glu should also be directed to subcortical nuclei such as the caudate, putamen, and thalamus, classically implicated in OCD.
Our second major finding was that pre-CBT vPCC Glu was higher in non-responders than in responders to CBT. The vPCC has been examined using MRS only once in (adult) OCD (Brennan et al, 2016); below-normal glutathione was found. Emerging findings in other neuroimaging modalities also possibly implicate the vPCC (Cheng et al, 2013; Tang et al, 2013; Ciesielski et al, 2012). The vPCC is thought to evaluate the self-relevance of an experience (Vogt, 2009). Accordingly, BOLD activation of vPCC has been observed in response to personalized obsession-inducing images in OCD (Morgiève et al, 2014). In evaluating the self-relevance of an experience, the vPCC is hypothesized to determine whether it will be felt as happy, sad, or neutral (‘emotional preprocessor’; Morgiève et al, 2014). If Glu levels index relevant vPCC functional activity, this may partly explain the association of higher vPCC Glu with reduced CBT response in this study.
The observed CBT-related reduction of Glu in pACC and predictive effect of vPCC Glu suggest that pharmacological interventions modulating glutamatergic systems may enhance CBT response in OCD, especially in partial responders or non-responders. Increasingly, Glu modulation is targeted for the development of novel OCD therapeutics, with many open-label reports and few controlled studies suggesting benefits of riluzole, N-acetyl-cysteine, memantine, D-cycloserine, topiramate, lamotrigine, ketamine, and sarcosine (Pittenger et al, 2011; Feusner et al, 2009). Interestingly, in relation to our findings, most of these agents are Glu receptor antagonists or downmodulators, while others act as partial Glu (NMDA) receptor agonists or upmodulators. The impact of these agents on OCD has been variable. Our findings encourage further experimentation but highlight that individual variation is considerable and that identification of Glu subgroups may be necessary when testing Glu-modulating agents.
Our report has limitations. Our experimental PEPSI MRS pulse-sequence required ~10 min runtime and multiple outer-volume saturation bands, leading to higher-than-desired rates of unsuccessful acquisitions and rejected data owing to motion and other artifacts, thus reducing power for planned analyses, especially for vPCC. Upgrades to PEPSI may mitigate these challenges. Our findings of changes in Glu with CBT are limited to short-term observations; whether these changes persist is unknown. As our Glu measures were snapshots pre- and post-CBT, we cannot identify when in the course of CBT pACC Glu reduction took place. Also, human in vivo MRS registers effects only at the whole tissue level (including summed intracellular and extracellular metabolite concentrations) and above. Hence, effects such as changes in Glu in presynaptic terminals or in the synaptic cleft may have escaped detection. These limitations notwithstanding, present results are generalizable at least to unmedicated pediatric OCD patients undergoing weekly CBT, an efficacious treatment without untoward effects. Studies of Glu during CBT and pharmacological manipulations may reveal more detailed mechanics of successful therapies and indicate more effective therapeutic strategies.
Funding and disclosure
This work was supported by the National Institutes of Mental Health grants R01MH081864 (to JON and JP) and R01MH085900 (to JON and JD Feusner), a UCLA Clinical and Translational Science Institute Award (to ELN), and International OCD Foundation Research Award 20153694 (to ELN). JP discloses research funding from Pfizer/DCRI, the Petit Foundation, Tourette Association of America, and the Trichotillomania Learning Center Foundation for Body-Focused Repetitive Behaviors; honoraria and travel from the International OCD Foundation and Tourette Association of America; and book royalties from Guilford Press and Oxford University Press. JTM has received consultant income from Think Now, honoraria from Alcobra Pharma for DSMB participation, research support from Psyadon, and study drug from AstraZeneca. The other authors declare no conflict of interest.
References
Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E et al (2009). Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res 172: 136–139.
Benazon NR, Moore GJ, Rosenberg DR (2003). Neurochemical analyses in pediatric obsessive-compulsive disorder in patients treated with cognitive-behavioral therapy. J Am Acad Child Adolesc Psychiatry 42: 1279–1285.
Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK (2009). Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology 34: 2489–2496.
Brennan BP, Jensen JE, Perriello C, Pope HG Jr, Jenike MA, Hudson JI et al (2016). Lower posterior cingulate cortex glutathione levels in obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 116–124.
Brennan BP, Rauch SL, Jensen E, Pope HG Jr (2013). A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biol Psychiatry 73: 24–31.
Brennan BP, Tkachenko O, Schwab ZJ, Juelich RJ, Ryan EM, Athey AJ et al (2015). An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacology 40: 1866–1876.
Carlsson ML (2000). On the role of cortical glutamate in obsessive-compulsive disorder and attention-deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr Scand 102: 401–413.
Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S (2005). Glutamatergic dysfunction in OCD. Neuropsychopharmacology 30: 1735–1740.
Cheng Y, Xu J, Nie B, Luo C, Yang T, Li H et al (2013). Abnormal resting-state activities and functional connectivities of the anterior and the posterior cortexes in medication-naive patients with obsessive-compulsive disorder. PLoS ONE 8: e67478.
Ciesielski KT, Rauch SL, Ahlfors SP, Vangel ME, Wilhelm S, Rosen BR et al (2012). Role of medial cortical networks for anticipatory processing in obsessive-compulsive disorder. Hum Brain Mapp 33: 2125–2134.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980.
Feusner JD, Kerwin L, Saxena S, Bystritsky A (2009). Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull 42: 81–93.
Fullana MA, Cardoner N, Alonso P, Subirà M, López-Solà C, Pujol J et al (2014). Brain regions related to fear extinction in obsessive-compulsive disorder and its relation to exposure therapy outcome: a morphometric study. Psychol Med 44: 845–856.
Geller DA (2006). Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am 29: 353–370.
Goodman WK, McDougle CJ, Barr LC, Aronson SC, Price LH (1993). Biological approaches to treatment-resistant obsessive compulsive disorder. J Clin Psychiatry 54: 16–26.
Hyder F, Fulbright RK, Shulman RG, Rothman DL (2013). Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J Cereb Blood Flow Metab 33: 339–347.
Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG (2006). Neuronal-glial glucose oxidation and glutamatergic-GSABAergic function. J Cereb Blood Flow Metab 26: 865–877.
Lázaro L, Bargallo N, Andrés S, Falcón C, Morer A, Junque C et al (2012). Proton magnetic resonance spectroscopy in pediatric obsessive-compulsive disorder: longitudinal study before and after treatment. Psychiatry Res 201: 17–24.
MacMaster FP, O'Neill J, Rosenberg DR (2008). Brain imaging in pediatric obsessive- compulsive disorder. J Am Acad Child Adolesc Psychiatry 47: 1262–1272.
McGuire J, Piacentini J, Lewin A, Brennan E, Murphy T, Storch E A meta-analysis of cognitive behavior therapy and medication for child obsessive compulsive disorder: treatment efficacy, response and remission. Depress Anxiety 32: 580–593 (2015).
Milad MR, Rauch SL (2012). Obsessive-compulsive disorder: beyond segregated cortico- striatal pathways. Trends Cogn Sci 16: 43–51.
Morgiève M, N'Diaye K, Haynes WI, Granger B, Clair AH, Pelissolo A et al (2014). Dynamics of psychotherapy-related cerebral haemodynamic changes in obsessive compulsive disorder using a personalized exposure task in functional magnetic resonance imaging. Psychol Med 44: 1461–1473.
Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C et al (2005). Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry 57: 901–910.
O'Neill J, Gorbis E, Feusner JD, Yip JC, Chang S, Maidment KM et al (2013). Effects of intensive cognitive-behavioral therapy on cingulate neurochemistry in obsessive-compulsive disorder. J Psychiatr Res 47: 494–504.
O'Neill J, Lai TM, Sheen C, Salgari GC, Ly R, Armstrong C et al (2016). Cingulate and thalamic metabolites in obsessive-compulsive disorder. Psychiatry Res 254: 34–40.
O'Neill J, Levitt JG, Alger JR (2013). Magnetic resonance spectroscopy studies of attention deficit hyperactivity disorder. In: Blüml S, Panigrahy A (eds). MR Spectroscopy of Pediatric Brain Disorders. Springer: New York, USA, pp 229–276.
O'Neill J, Piacentini JC, Chang S, Levitt JG, Rozenman M, Bergman L et al (2012). MRSI correlates of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 36: 161–168.
Ortiz AE, Ortiz AG, Falcon C, Morer A, Plana MT, Bargalló N et al (2015). 1H-MRS of the anterior cingulate cortex in childhood and adolescent obsessive-compulsive disorder: a case-control study. Eur Neuropsychopharmacol 25: 60–68.
Piacentini JLA, Roblek T (2007) Overcoming Childhood OCD: A Therapist’s Guide. Oxford University Press: New York, USA.
Piacentini J, Bergman RL, Chang S, Langley A, Peris T, Wood JJ et al (2011). Controlled comparison of family cognitive behavioral therapy and psychoeducation/relaxation training for child obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 50: 1149–1161.
Pittenger CBM, Wegner R, Teitelbaum C, Krystal JH, Coric V (2006). Glutamate dysfunction in obsessive-compulsive disorder and the potential clinical utility of glutamate-modulating agents. Primary Psychiatry 13: 65–77.
Pittenger C, Bloch MH, Williams K (2011). Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther 132: 314–332.
Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG et al (2007). Proton echo- planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med 58: 236–244.
Rosenberg DR, Keshavan MS (1998). AE Bennett Research Award. Toward a neurodevelopmental model of obsessive–compulsive disorder. Biol Psychiatry 43: 623–640.
Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ (2000). Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry 39: 1096–1103.
Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP et al (2004). Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry 43: 1146–1153.
Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL (2011). 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed 24: 943–957.
Saxena S, Gorbis E, O'Neill J, Baker SK, Mandelkern MA, Maidment KM et al (2009a). Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder. Mol Psychiatry 14: 197–205.
Saxena S, O'Neill J, Rauch SL (2009b). The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt B (ed). Cingulate Neurobiology and Disease. Oxford University Press: New York, USA, pp 587–618.
Seese RR, O'Neill J, Hudkins M, Siddarth P, Levitt J, Tseng B et al (2011). Proton magnetic resonance spectroscopy and thought disorder in childhood schizophrenia. Schizophr Res 133: 82–90.
Simpson HB, Shungu DC, Bender J Jr, Mao X, Xu X, Slifstein M et al (2012). Investigation of cortical glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology 37: 2684–2692.
Starck G, Ljungberg M, Nilsson M, Jonsson L, Lundberg S, Ivarsson T et al (2008). A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J Neural Transm (Vienna) 115: 1051–1062.
Tang W, Li B, Huang X, Jiang X, Li F, Wang L et al (2013). Morphometric brain characterization of refractory obsessive-compulsive disorder: diffeomorphic anatomic registration using exponentiated Lie algebra. Prog Neuropsychopharmacol Biol Psychiatry 46: 126–131.
Torp NC, Dahl K, Skarphedinsson G, Compton S, Thomsen PH, Weidle B et al (2015). Predictors associated with improved cognitive-behavioral therapy outcome in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 54: 200–207.
Vogt B (2009) Regions and subregions of the cingulate gyrus. In: Vogt B (ed). Cingulate Neurobiology and Disease. Oxford University Press: New York, USA. pp 3–30.
Whiteside SP, Abramowitz JS, Port JD (2012). Decreased caudate N-acetyl-l-aspartic acid in pediatric obsessive-compulsive disorder and the effects of behavior therapy. Psychiatry Res 202: 53–59.
Wu K, Hanna GL, Rosenberg DR, Arnold PD (2012). The role of glutamate signaling in the pathogenesis and treatment of obsessive–compulsive disorder. Pharm Biochem Behav 100: 726–735.
Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J et al (2008). Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust NZ J Psychiatry 42: 467–477.
Zhang J (2013). Human brain glutamate, glutamine, γ-aminobutyric acid, proton magnetic resonance, spectral quantification with the fast Padé transform. Doctoral dissertation, University of California, Los Angeles, CA, USA.
Zurowski B, Freyer T, Wahl K, Büchert M, Kuel AK, Hohagen F et al (2007). Neurochemical abnormalities in patients with obsessive-compulsive disorder diminish in the course of behavior therapy. Soc Neurosci Abstr 450: 10.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
O'Neill, J., Piacentini, J., Chang, S. et al. Glutamate in Pediatric Obsessive-Compulsive Disorder and Response to Cognitive-Behavioral Therapy: Randomized Clinical Trial. Neuropsychopharmacol 42, 2414–2422 (2017). https://doi.org/10.1038/npp.2017.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.77